Federated learning scales real-world data access for external control arms
Our research on federated external control arms (FedECA) has just been published in Nature Communications! This work marks a major milestone for privacy-preserving clinical research and international collaboration in cancer trials.
The clinical development of a new drug passes through three distinct phases imposed by regulatory agencies and the whole cycle requires 12 years on average to complete. Oncology trials suffer from poor success rates with more than 80% of cancer therapies in phase III trials failing to meet their primary endpoint. This failure rate has a cascade of negative effects across healthcare systems as clinical trials are very expensive and the costs are indirectly passed on to patients and hospitals. Increasing success rates would not only lower costs of drug development but potentially also accelerate the pace at which new drugs can reach patients and improve their lives.
Phase I and II trials primarily aim to measure the maximum tolerated dose and the safety of the drug, not the efficacy of a drug’s benefits for patients. One of the major reasons why trials miss their endpoints is not catching potential issues in these earlier phases. This could be improved by building a Phase “2.5” using real world data in an external control arm analysis, which would help prevent wasting time and patient chances in Phase III that we know will fail and provide intermediate evidence to the regulators for promising new drugs before phase III.
Randomized clinical trials (RCTs) are the gold standard for evaluating the causal effect of a drug. Traditional clinical trials use concurrent control arms, where participants are randomly assigned to either the experimental group (receiving the new drug) or the control group (receiving a placebo or current standard-of-care treatment). However, in practice, it can be challenging to recruit enough patients to conduct a concurrent control group, especially when conducting rare disease trials or using a drug that targets specific patient subpopulations. Researchers can overcome this challenge by leveraging external control arms, which use other patient data to mimic the characteristics of a concurrent control arm.
Federated learning unlocks data access to fuel external control arms
Unfortunately, it is often hard to obtain enough data to power external control arms as it is distributed in different medical institutions and subject to regulations such as GDPR and HIPAA which makes pooling the data significantly more complex from a contractual and logistical perspective. In order to access diverse and representative data for use in external control arms there is a need for privacy-enhancing technologies (PET) to protect both patient confidentiality and competitive advantage for pharmaceutical companies. One such PET - federated learning - enables researchers to harness the knowledge from several institutions while leaving patients’ data on premises by only sharing mathematical quantities known as gradients instead of the raw data. This technology allows researchers to perform data analysis while facilitating compliance with regulations and protecting intellectual property (IP) and data ownership.
Watch the video to learn how federated learning works
Owkin scientists have investigated using recent advances in federated learning in combination with ECA techniques to create different methodologies for distributed external control arms with time-to-event outcomes, while limiting patient data exposure. Inverse probability of treatment weighting (IPTW) is a popular ECA method used to tap into real-world data or historical clinical trials to build a control group for a single-arm trial.
Our research introduces a federated learning IPTW method for time-to-event outcomes, which we call FedECA.
FedECA extends WebDISCO - an existing method permitting federated learning of time-to-event models in ECA contexts, by integrating propensity scoring and causal inference to the learning process. We illustrated the feasibility of this approach by performing experiments on simulated and real data using Substra, an open source federated learning technology with proven experience in privacy-sensitive contexts in healthcare.
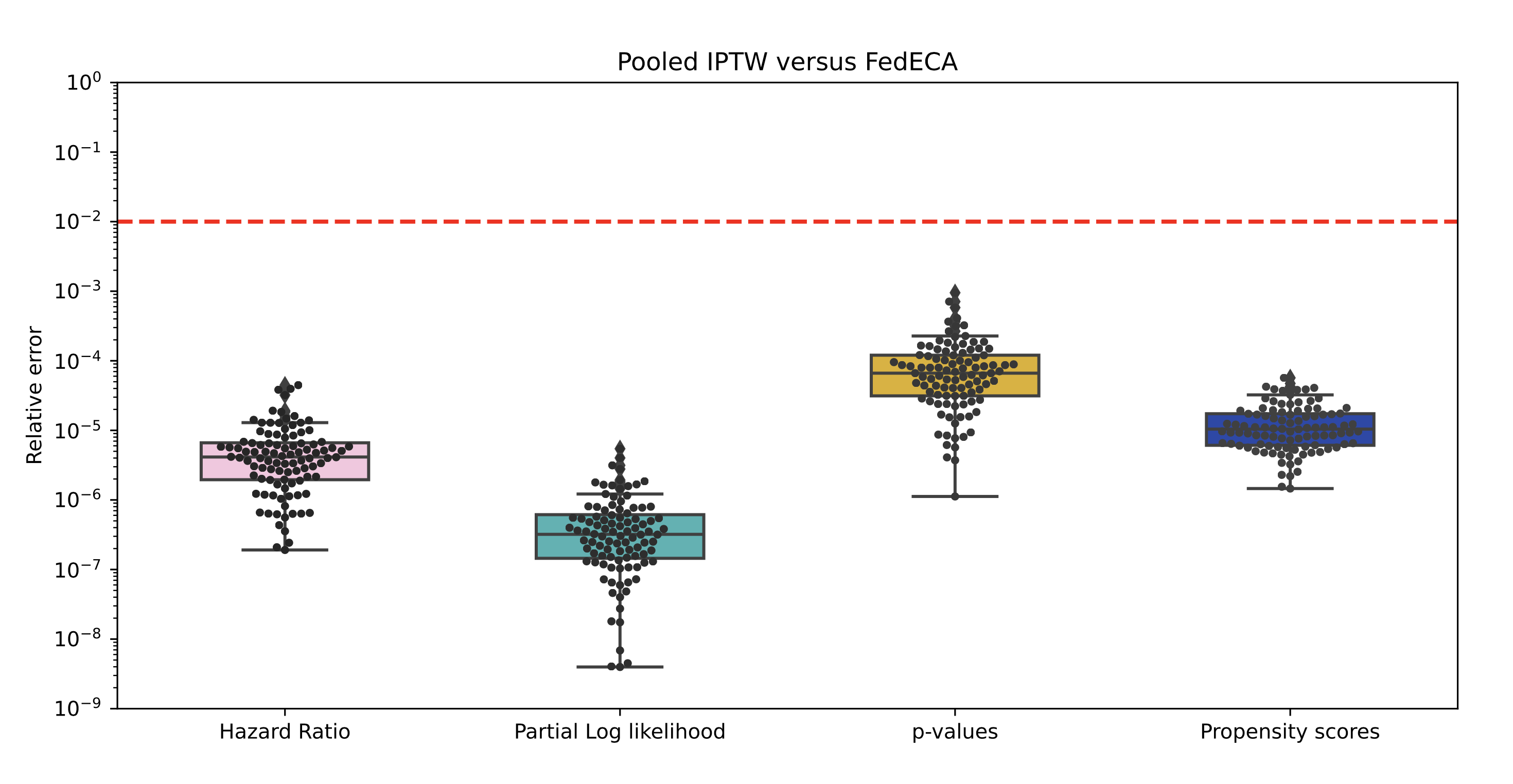
Our results demonstrated that FedECA is equivalent to the commonly used IPTW method on pooled data and that it even outperforms federated analytics counterparts in specific contexts. We have made code publicly available with unrestricted use for researchers to reproduce the analyses included in the paper and invite the community to contribute. We also explore the use of differential privacy in order to limit potential privacy leakage induced by the repeated sharing of risk sets and its impact on the performance of the analysis.
FedECA was recently implemented in a real-world federated network across three leading cancer centers—FFCD (France), IDIBGI (Spain), and PanCAN (USA). This enabled, for the first time, a fully federated external control arm analysis comparing the effectiveness of two first-line treatments for metastatic pancreatic cancer using real patient data from international centers, all while preserving patient privacy and regulatory compliance.
To see the full results, read the paper here.
Jean du Terrail, first author of this research paper said:
By leveraging the latest developments in federated learning (FL) research and open-source softwares, it is now possible to build distributed data analysis pipelines that are both as easy to use and produce results of similar quality as one would get if one could pool all the data in a single place, which is not often not feasible in practice. While there is still a lot to be done on the research side to strengthen the privacy of such analyses even further, the FL community is continuously making progress on that ground and Owkin is proud to contribute to that open and collaborative effort.
Improving external control arms to de-risk and accelerate clinical trials
External control arms are a promising solution to improve clinical trial outcomes and FedECA can be a powerful approach to unlock the data from multiple sources needed to make them more effective in real world settings. Reducing the number of failed oncology trials could greatly positively impact the pharmaceutical industry, healthcare systems and ultimately patients. By increasing scientific and clinical accuracy, biopharma companies can also improve investor and regulatory perception of their assets. Owkin are experts in both clinical trial optimization and the application of federated learning in healthcare settings, and can help organizations navigate new approaches in this rapidly changing landscape.




