
AI Diagnostics
About our products
Clinically validated digital pathology AI to augment precision oncology workflows
Transform image into action with AI diagnostics
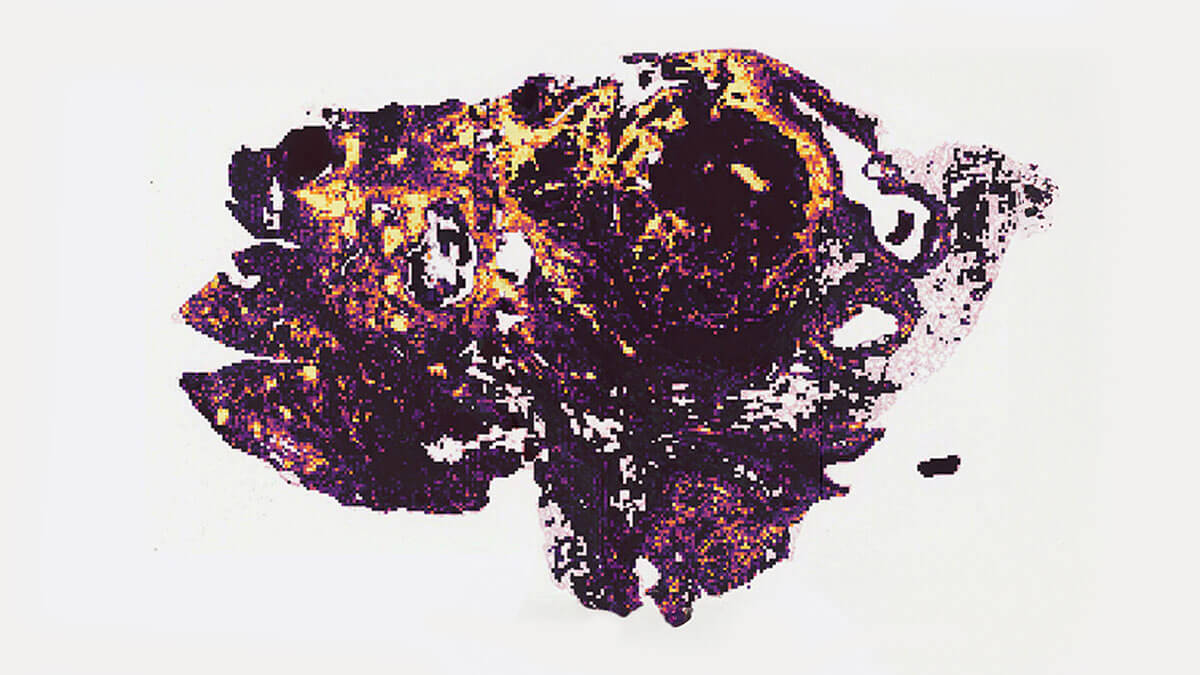

H&E
Lymphocytes

Pictured here: RlapsRisk BC results in action. Image for presentation only.
First-in-class
Groundbreaking AI diagnostics, accessible across diverse workflows and IT settings
Explore our products
Our portfolio consists of products for clinical trials or research settings, and IVD solutions that are regulatory-approved and ready for use in clinical routine.
Thank you! Your submission has been received!
Oops! Something went wrong while submitting the form.
*Certain products are labeled Research Use Only (RUO) based on regional regulatory requirements.
How can you use our AI Dx?

Pharma
Match patients to approved drugs in clinical routine
Discover more


Pharma
Accelerate patient identification for clinical trial enrolment
Discover more


Clinicians & Labs
Optimize lab workflows with pathologist enhancements and case prioritization
Discover more


Clinicians & Labs
Drive therapeutic decision making to improve patient outcomes
Discover more

From data to approved medical device, we lead the field in AI diagnostic development

Best Medical Technology
This award highlights Owkin’s dedication to enhancing the efficacy and precision of medical treatments, propelling them as a leader in the healthcare sector.
Read more


Innovation -
AI Diagnostics
For integrating artificial intelligence to enhance diagnostic efficiency and accuracy, revolutionizing the traditional pathology workflow.

Product Launches - AI Diagnostics
For significantly streamlining the diagnostic process by pre-screening, reducing the number of patients requiring standard MSI testing.

3 Stevie® Awards
Technology Breakthrough in Artificial Intelligence
Technology Breakthrough in Healthcare Technology
Grand Stevie for Highest-Rated Nomination of the year
Our building blocks for robust diagnostics
Data
Our vast patient data network lays the foundation for impactful products that meet critical medical needs.
AI & data science
We are a world leader in AI and machine learning for digital pathology.
Regulatory and quality standards
Owkin has regulatory profficiency in navigating the new CE-IVDR and FDA frameworks.
Images shown may represent the range of products, or be for illustration purposes only, and may not be an exact representation of the product.
Information last updated on 18th March 2025.



_Destra%20Wordmark.png)








